ISSN: 2320-2459
ISSN: 2320-2459
T Karunakara, CH Srinivasub, K Narendrac*
1Department of Physics, NRI Academy College, Vijayawada, Andhra Pradesh, India
2Department of Physics, Andhra Loyola College, Vijayawada, Andhra Pradesh, India
3Department of Physics, VR Siddhartha Engineering College, Vijayawada, Andhra Pradesh, India
Received date: 01/10/2012 Revised date: 10/11/2012 Accepted date: 16/12/2012
Visit for more related articles at Research & Reviews: Journal of Pure and Applied Physics
Ultrasonic velocity and density measurements for binary mixtures of aniline + 1-butanol at T= (303.15 – 318.15) K, are conducted at atmospheric pressure. Some thermo acoustical parameters like adiabatic compressibility (β), molar volume (Vm), inter molecular free length (Lf) and acoustic impedance (Z) have been calculated from experimental measurements. The results have been used qualitatively to explain the molecular interaction between the components of these mixtures. Further, the results are further supported by FT-IR spectra
Ultrasonic velocity, acoustic parameters, aniline, 1-butanol, density, IR Spectra
In recent years, measurements of ultrasonic velocities have been used to understand the nature of molecular interactions in pure liquids and liquid mixtures in general and binary mixtures in particular. The study of thermodynamic parameters in binary liquid mixtures by measuring the ultrasonic velocity and density as a function of mole fraction as well as temperature has drawn the attention of various research groups for the last several years.
In view of the availability of simple and accurate velocity measurement techniques, these ultrasonic studies of binary liquid mixtures also entered into new and diverse fields such as medical imaging, Brilliouin scattering spectroscopy, biomedical research which needs the propagation of ultrasonic waves without causing much attenuation to the signal.
Properties of liquid-liquid mixtures are thermodynamically very important as a part of studies of thermodynamic, acoustic and transport aspects. The compositional dependence of thermodynamic properties is proved to be very useful tool in understanding the nature and extent of pattern of molecular aggregation resulting from intermolecular interaction between components. This type of study is a powerful means of characterizing the various aspects of physico-chemical behavior of liquid mixtures and studying the interaction between the molecules.
Further, ultrasonic velocities, densities and derived thermodynamic and acoustical parameters are of considerable interest in understanding the intermolecular interactions in binary [1, 2] liquid mixtures. Volumetric, acoustic and thermodynamic studies in mixtures have been used for understanding the intermolecular interactions by many researchers for interpreting different types of interactions viz., dipole-dipole [3, 4] and dipole-induced dipole [5, 6] in polar-polar [7, 8] and in polar-non polar [9, 10] systems. Increasing use of benzene, toluene and 1-butanol in many industrial processes have greatly stimulated the need for extensive information on the acoustic and transport properties of these liquids and their mixtures. 1-Butanol is used as a blending agent for petroleum, naphtha, resins and gums and also as an extractant in the production of vitamins, hormones, essential oils, antibiotics and sulphonated oils [11].
In an attempt to explain the nature of interactions occurring between Aniline+1-Butanol, ultrasonic velocity and density of binary liquid mixtures have been determined over the entire range of composition at T = (303.15, 308.15, 313.15, and 318.15) K in the present study. Using the experimental values of u and ïÃÂò, values of adiabatic compressibility, ïÃÂâ, molar volume, Vm, intermolecular free length, Lf, acoustic impedance, Z, have been calculated. In the light of these parameters and results from IR spectra, the intermolecular interactions have been estimated [12].
Experimental Procedure
To prepare the mixtures of required proportions Job’s method of continuous variation was used. The mixtures were preserved in well-stoppard conical flasks. After mixing the liquids thoroughly, to allow them to attain thermal equilibrium the flasks were left undisturbed.
The ultrasonic velocities were measured by using single crystal ultrasonic pulse echo interferometer (Mittal enterprises, India; Model: F-80X). The measurements of ultrasonic velocity were made at a fixed frequency of 3MHz. It consists of a high frequency generator and a measuring cell. By measuring the velocity in carbontetrachloride and benzene calibration of the equipment was done. The results are in good agreement with those reported in literature [13]. The accuracy maintained in the measurement of ultrasonic velocity is ± 0.5 ms-1. By circulating water around the liquid cell from thermostatically controlled constant temperature water bath of accuracy ± 0.01K the temperature was controlled.
By using a specific gravity bottle with an accuracy of ± 0.5% the densities of pure liquids and liquid mixtures were measured. Averages of 4-5 measurements were taken for each sample. An electronic balance (Shimadzu AUY220, Japan), with a precision of ±0.1 mg was used for the mass measurements.
Theory
From the experimental data of ultrasonic velocity and density various thermo acoustic parameters are calculated using standard equations mentioned below.
Adiabatic compressibility
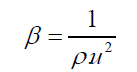 (1)
(1)
Molar Volume
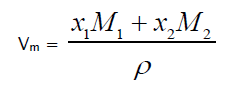 (2)
(2)
Where M1 and M2 are the molecular weights of constituent components.
Intermolecular free length
![]() (3)
(3)
Where KT is the temperature dependent constant [14].
Acoustic impedance
![]() (4)
(4)
The experimental values of ultrasonic velocity (u) and density (ïÃÂò) of the mixture of Aniline + 1-Butanol over the entire composition range and at temperatures T = (303.15, 308.15, 313.15 and 318.15) K are given in Table 1. The calculated values of adiabatic compressibility (β), molar volume (Vm), inter molecular free length (Lf) and acoustic impedance (Z) at different temperatures is presented in Tables 2 and 3. The IR Spectra of binary mixture at five different mole fractions are shown in Figures 1 to 5. From Table 1 it is observed that as the mole fraction increases the ultrasonic velocities decreases at all temperatures, the decrease in velocity suggest the weak molecular interaction between aniline and 1-butanol.
From Table 2, it is observed that adiabatic compressibility increases with increasing concentration of 1-butanol. It is primarily the compressibility that changes with molecular structure which leads to change in ultrasonic velocity. The change in β in liquid mixtures indicates that there is a definite contraction on mixing and the variation may be due to complex formation. This clearly shows that there are some significant interactions between the molecules of the mixture taken up for study. It is observed from the same table the changes in molar volume with mole fraction. The molar volume increases with rise in temperature in the present study, which may probably would be caused from the fact that thermal energy facilitates an increase in the molecular separation in the liquid mixtures which leads to an increase in molar volume (Vm) with increase of temperature. It is also observed that adiabatic compressibility values and molar volumes increase with temperature at a particular mole fraction.
The increase in compressibility keeps the molecules to a large distance resulting into an increase in intermolecular free length as observed in Table-3. Intermolecular free length is a predominant factor in determining the variation of ultrasonic velocity. As Lf increases u decreases and vice-versa [15]. As an acoustic wave travels in a medium, there is a variation of pressure from particle to particle. Acoustic impedance is governed by the inertial and elastic properties of the medium. In the present system, it is observed that these values decrease with increasing concentration of 1-butanol. Such a decrease in values of Z further supports the presence of weak molecular interactions between the molecules of aniline and 1-butanol.
Generally the intensity of an absorption in the IR spectrum is related to the change in dipole moment that occurs during the vibration. Consequently, vibrations that produce a large change in dipole moment (e.g. C=O stretch) result in a more intense absorption than those that result in a relatively modest change in dipole (e.g. C=C). Vibrations that do not result in a change in dipole moment (e.g., a symmetrical alkyne C ≡ C stretch) will show little or no absorption for this vibration.
The present mixture shows very small variations in their intensity of the respective bonds i.e., dipole moment and hence it supports that weak interactions take place in the mixture of aniline +1-butanol.
From the data of ultrasonic velocity and density various acoustical parameters of the mixtures of aniline with 1-butanol at 303.15, 308.15, 313.15 and 318.15 K were calculated. It is observed that there exist weak molecular interactions between the unlike molecules. It is also supported by the IR spectra.