e-ISSN: 2321-6182 p-ISSN: 2347-2332
e-ISSN: 2321-6182 p-ISSN: 2347-2332
Jayeola CO1* Oluwadun A2, Olubamiwa O1, Deji Agboola2, and Effedua HI2
1Crop Processing and Utilization Division, Cocoa Research Institute of Nigeria, P.M.B., 5244, Ibadan, Oyo State, Nigeria
2Dept of Medical Microbiology and Parasitology, Obafemi Awolowo College of Health Science, Olabisi Onabanjo University Sagamu, Ogun State, Nigeria
Received: 03/03/2014 Revised: 17/03/2014 Accepted: 26/03/2014
Visit for more related articles at Research & Reviews: Journal of Pharmacognosy and Phytochemistry
Obesity and overweight have in the last decade become a global health problem - according to the World Health Organization (WHO) back in 2005 approximately 1.6 billion adults over the age of 15+ were overweight, at least 400 million adults were obese and at least 20 million children under the age of 5 years were overweight. Experts believe if the current trends continue by 2015 approximately 2.3 billion adults will be overweight and more than 700 million will be obese. The scale of the obesity problem has a number of serious health consequences for individuals and government systems. This study was conducted to determine the anti -obese activity of cocoa powder through the use of mouse model. Natural cocoa powder was included in the composition of mouse feed. The cocoa feed was fed to group of obese mice, normal /un-obese mice and compared with their counterpart using normal mice feed over a period of two weeks. The mean percentage weight reduction was more pronounced in the obese mice when the weight reduction was compared with the normal mice. Mice weight of 17.60+1.27g before administration of cocoa, 16.65 + 1.63g after first week of treatment and 15.60+0.00g after second week of treatment showed constant reduction on the weight of mouse fed with cocoa powder. This is an indication that there was systematic decline in weight of mice after being fed with cocoa for a period of two weeks; this demonstrated that cocoa may contain some weight trimming ingredients.
cocoa powder, weight reduction, obese mice
Obesity results from energy imbalance between energy intake and energy expenditure over a period of time. Increased energy intake (calories) with the decline of physical activity promotes weight gain, body fat storage and adiposity growth in a pathologic direction [10]. Studies have shown that the obesity contributes to an increasing risk of major depression, emotional disorders, early death, disability, menstrual disorders, infertility, miscarriage, and poor pregnancy. It is also concluded that there is strong evidence that obesity is associated with increased morbidity and mortality. Obesity is a concern because of its implications for the health of an individual as it increases the risk of many diseases and health conditions including: -
Hypertension, Dyslipidemias (high total cholesterol or high levels of triglycerides),
Type 2 diabetes, Coronary heart disease, Metabolic syndrome, Stroke, liver and Gallbladder disease, Osteoarthritis(a degeneration of cartilage and its underlying bone within a joint), Sleep apnea and respiratory problems and Some cancers (endometrial, breast, and colon) and Gynaecological problems (abnormal menses, infertility). These conditions can cause or contribute to premature death and substantial disability [5,6,7]. Cardiovascular disease - mainly heart disease and stroke - is already the world's number one cause of death, killing 17 million people each year and diabetes has rapidly become a global epidemic - according to WHO projections diabetes deaths will increase by more than 50% worldwide in the next 10 years.
Even though, the consequence of obesity is so severe, there is not an effective method to prevent and treat obesity. We have, therefore, dedicated ourselves to develop a more successful method to prevent obesity. Although obesity is associated to increase of body weight, the definition of obesity is not dependent on body weight but on the amount of body fat, specifically adipose tissue. In other word, obesity is a condition of abnormal large amount of fat stored in adipose tissue and an increase in bodyweight is generally associated with an increased risk of excessive fat-related metabolic diseases (EFRMD) and chronic diseases, including Type 2 diabetes mellitus, hypertension and dyslipidemia [2,3].
Cocoa a rich source of polyphenol substances, such as (–)-epicatechin (EC), (+)-catechin, quercetin (including its glucoside), clovamide, deoxyclovamide, trans-resveratrol and its glucoside (trans-piceid) and procyanidin [8]. Over the past decade, at least 28 studies have been reported on the health benefits of cocoa flavonoids [4]. Flavonoids represent a group of molecules of increasing interest. The major flavonoid is Quercetin, which belongs to the class called flavonols and is mainly found in apples, tea, onions, nuts, berries, cauliflower, cabbage and many other foods. It exhibits a wide range of biological functions including anticarcenogenic, anti-inflammatory and antiviral; it also inhibits lipid peroxidation, platelet aggregation and capillary permeability. This review focuses on the main effects of Quercetin on obesity and diabetes. The mechanisms of action explaining the effects of Quercetin on these two metabolic disturbances are also considered. Good perspectives have been opened for Quercetin, according to the results obtained either in cell cultures or in animal models. Nevertheless, further studies are needed to better characterize the mechanisms of action underlying the beneficial effects of this flavonoid on these pathologies. Moreover, the body fat-lowering effect and the improvement of glucose homeostasis need to be confirmed in humans. Animal studies have consistently failed to demonstrate adverse effects caused by Quercetin. In contrast, due to inhibitory effect of Quercetin in cytochrome P450, interactions with drugs can be taken into account when they are administered at the same time than Quercetin. It has been reported catechins, especially EGCG, have anti-obesity effect in mice by lowering the food intake and preventing body weight gain [11,12]. Cocoa powder could be used as dietary functional ingredients in the fight against obesity.
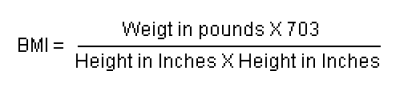
BMI provides a benchmark for individual assessment, but experts suspect that the risk of chronic disease in populations increases progressively from a BMI of 21 upwards.
Other methods of estimating body fat and body fat distribution include measurements of skinfold thickness and waist circumference, calculation of waist-to-hip circumference ratios, and techniques such as ultrasound, computed tomography, and magnetic resonance imaging (MRI).
This experiment made use of mouse model to determine the anti-obesity property of cocoa powder. Laboratory mice have been the most important non-human models for studying the effectiveness of new drug therapies.
Male and female Naïve BALB/C mice of 8-12 weeks old (14-22g) for normal mice and (24-26g) for obese mice were used for this study and they were purchased from Akintola farm (a commercial animal breeding house) in Ibadan Oyo State, Nigeria.
Natural flavanol-rich cocoa powder (non-alkalized) which was produced by an innovative industrial process and packaged by Cocoa Research Institute of Nigeria (CRIN), Idi-Ayunre, Ibadan, Oyo State, Nigeria, was used for this experiment. This is to evaluate its functionality in a short-term study through the use of an experimental rodent model of anti-obesity.
The experiment was conducted at the laboratory of the department of pharmacology, Obafemi Awolowo College of Health Sciences, Olabisi Onabanjo University, Sagamu, Ogun State, Nigeria
The experimental feed was specially formulated on request to be made of the normal rat diet with inclusion of 2% natural cocoa powder. The modified feed consisted of maize starch, sucrose, soybean oil, fibre (cellulose powder), mineral premix, choline bitartrate, tert-butyl-hydroquinone and 2% cocoa powder [9]. This was made into rat feed pellet by Pfizer feed mill, Iwo road, Ibadan. The diet contained (g/kg): maize starch, 397·486; casein, 200·000; dextrinised maize starch, 132·000; sucrose, 100·000; soyabean oil, 70·000; fibre (cellulose powder), 50·000; cocoa powder, 20.000; AIN-93G mineral mix, 35·000; AIN-93 vitamin mix, 10·000; L-cystine, 3·000; choline bitartrate, 2·500; tert-butylhydroquinone, 0·014.
Mice were housed in polypropylene cages maintained at standard condition (12hours light/dark cycle 25± 3°C, 45-65% humidity). The animals had free access to modified standard mouse feed and water ad libitum. All the animals were acclimatized to laboratory condition for 3days before commencement of the experiment as described by Karunakar et al, (2009).
Experimental mice were grouped into four groups (A to D) randomly containing 10 animals each, according to their weight and sex. Each group comprises of both male and female mice.
Group A – normal mice fed with normal rat feed
Group B – obese mice fed with normal rat feed
Group C – normal mice fed with compounded cocoa feed
Group D – obese mice fed with compounded cocoa feed
Weights of the experimental mice were taken before the commencement of the experiment (i.e day 0) and on days 2, 4, 6, 8, and 14 days. The mean weights were taken and percentage weight loss or gain was determined through comparison of the mean weight before and after the experiment.
The values obtained were expressed as the mean ± SE (standard error). Statistical comparisons of two groups were determined by the Student’s t-test. Statistical significance was defined as p<0.05.
The initial mean weights of experimental mice were shown in Table 1. The different groups were constituted based on the weight of the mice, the mean weight for normal mice ranged between 17.60-17.64g while that of the obese mice was between 24.45 – 26.50g. the comparative mean weight of all the experimental animals were taken at the end of the experiment, it was observed that there was an increase in the weight of obese mice mice fed with their normal diet while we recorded a significant (p<0.05) decreasing trend in normal and obese mice fed with cocoa compounded feed as shown in Table 2. It was observed during the experiment that mice fed with cocoa compounded feed reduce their food intake when compared with those on their normal diet though very active.
The effect of cocoa consumption on mice weight was determined in Table 3, a systematic decline was observed in the weight of mice with mean weight of 17.60±1.27g before administration of cocoa, 16.65 ± 1.63g after first week of treatment and 15.60±0.00g after second week of treatment. The difference was however, found to be insignificant (F = 1.41, P > 0.05). Similarly, the same trend was observed on obese mice but there was a significant decrease (F=22.31, P<0.05) in the weight of obese mice from 26.50 ± 1.44 to 21.22 ± 1.34g as depicted in Table 4.
The assessment of mean weight reduction on obese male and female mice fed with cocoa compounded feed as shown in Table 5 indicated that there was no significant difference in the weight reduction observed in mice from both gender ( t = 0.61, P > 0.05 ). Moreover, the systematic decline in weight of mice after being fed with cocoa for a period of two weeks demonstrated that cocoa may contain some weight trimming ingredients. This is in agreement with the studies carried out on the use of quercetin a major flavonoid in cocoa in addressing the potential anti-obesity action of mice in vitro, as carried out by Ahn, et al., [1]. He showed that Quercetin decreased the expression of Sterol Regulatory Element-Binding Proteins (SREBP)-1 and Fatty Acid Synthase (FAS), and by increasing Acetyl-CoA Carboxilasa (ACC) phosphorylation. Also in another study, Rivera et al., [15] analysed the effect of a chronic administration of Quercetin (2 or 20 mg/kg body weight/d) in obese Zucker rats (a model of genetic obesity). In good accordance with Steward et al.,[16], final body weight was decreased. Using C57BL/6J mice several authors have found reductions in body fat induced by Quercetin treatment. Liang et al. [14] reported that mice fed on a HFD supplemented with Quercetin (66 mg/kg body weight/d) were protected against weight gain induced by the diet. Moreover, Kobori et al., [13] reported that chronic dietary intake of Quercetin reduced body weight gain, as well as visceral and liver fat accumulation, and improved systemic parameters related to metabolic syndrome (hyperglycemia, hyperinsulinemia and dyslipidemia), probably by decreasing oxidative stress and increasing PPAR expression. This study thereby agrees with above authors in that cocoa powder which contains a significant amount of quercertin had resulted into weight reduction in mice.
There has not been effective drug to treat obesity because they all have undesirable side effects. However, it is believed that botanicals and natural foods like cocoa powder provide a safer and natural way to human body in both pharmaceutical and nutraceutical aspect. In the search for new drug that could be used as dietary functional ingredients in obesity good perspectives have opened up for cocoa powder according to the results obtained in animal models.
Nevertheless, further studies are needed to better characterize the mechanisms of action underlying the beneficial effects of this flavonoid on obesity. Moreover, the body fat-lowering effect of cocoa powder needs to be confirmed in humans.
The outcome of this study showed that there is a good prospect for cocoa powder which is a food drink to be used as a drug that can prevent and treat obesity in the nearest future. I thereby recommend consumption of cocoa to people that have been implicated to be obese.
We are grateful to the Executive Director of Cocoa Research Institute of Nigeria Prof. M. Akoroda for the permission given to publish this paper.