
Page 44
Notes:
conferenceseries
.com
November 13-15, 2017 | Las Vegas, USA
14
th
International Conference and Exhibition on
Materials Science and Engineering
RRJOMS | Volume 5 | Issue 7 | November, 2017
Thermal-structural and elemental analysis of Cu-Fe-O system coupled with available thermodynamic
modeling
Juliano Schorne Pinto, Laurent Cassayre
and
Antoine Barnabé
CIRIMAT Université de Toulouse UPS, France
T
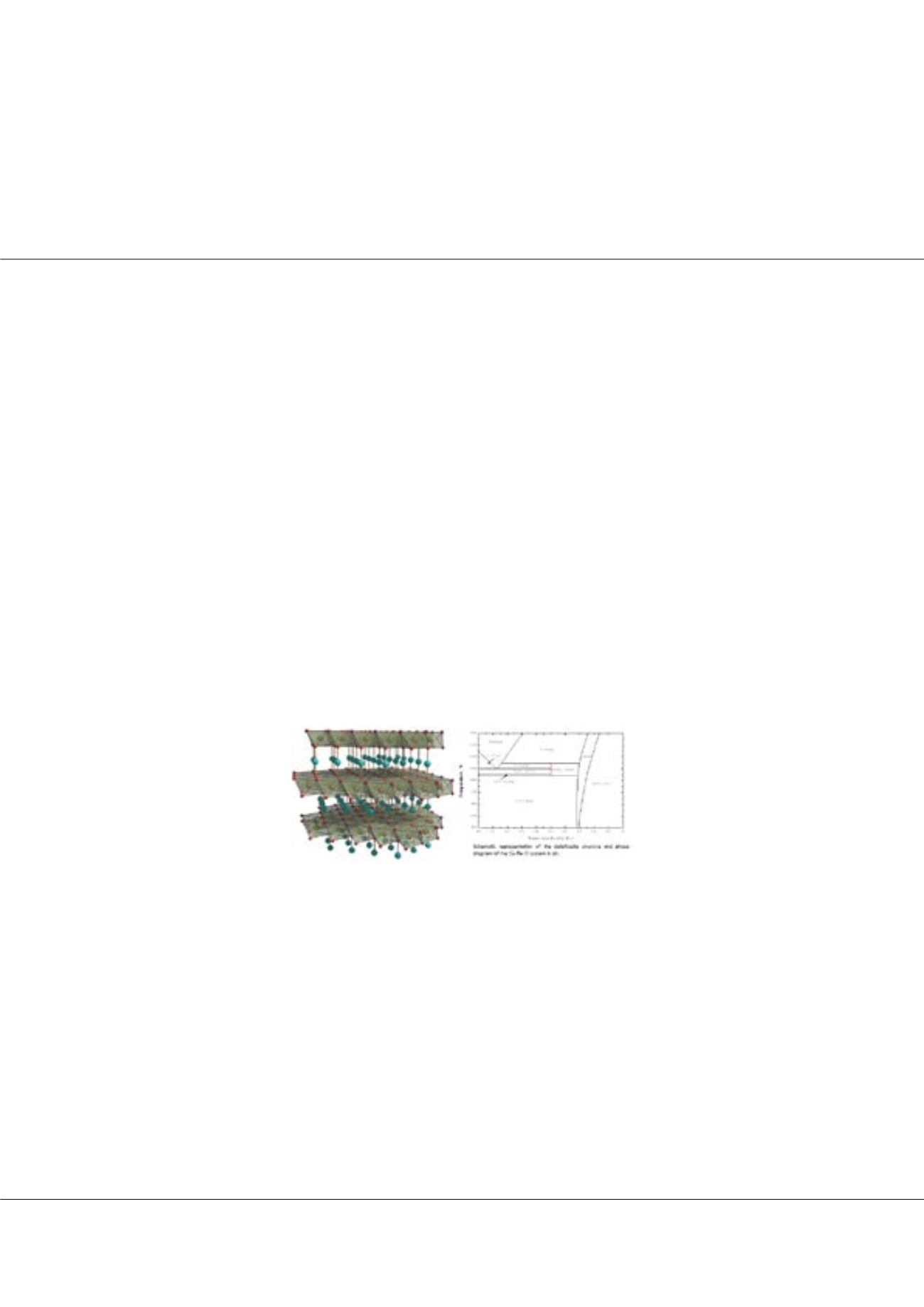
he Cu-Fe-O system has a great technological interest in the copper industry, as well as the development of catalytic compounds
and transparent devices. The CuFeO
2
phase (delafossite) and Cu
x
Fe
3-x
O
4
phase (spinel) exhibit remarkable electrical, magnetic,
optical and optoelectrical properties. Therefore, an in-depth understanding of the stability of the delafossite structure becomes of
particular interest for fundamental research and for instance, its applications to the development of efficient p-type TCOs. The
purpose of this study is reviewed the structural and thermodynamic information and phase equilibria of the Cu-Fe-O system in
addition to checking the consistency of the available thermodynamics models with the experimental data. First, several of these
models based on the CALPHADmethod were reviewed and differences were highlighted. Moreover, several experimental procedures
were employed to establish the relationships among temperature, lattice parameter, and stoichiometry of mixed oxides. In situ HT-
XRD (High-temperature X-Ray Diffraction) and TGA/DTA measurements, Rietveld refinement were used to provide thermo-
structural information in the range of 50° to 1100°C from stoichiometric mixture of CuO and Fe
2
O
3
single oxides. Plasma Sintering
(SPS) followed by adjusted post-annealing treatments were used to stabilize delafossite phase in different Copper/Iron gradient and
analyzed by Electron Probe Micro-Analyzer (EPMA). The HT-XRD demonstrated that the spinel phase started to be formed from
750° and increases the amount of Cu after 900°C (Cu
x
Fe
3-x
O
4
). In addition, the variation of lattice parameters of spinel phase was
determined by Rietveld refinement and compared with those of different molar ratios. Contrary to all the models, EPMA coupled
with local structural analysis showed that delafossite phase could be stabilized with a substantial degree of cationic non-stoichiometry.
These results were related to available thermodynamics models providing an improved understanding of this system, new information
has generated to implement the existing data. The need to develop and improve a new model is considered.
Biography
Juliano Schorne Pinto is a Ph.D. student at Université de Toulouse and research associate at the CIRIMAT and Laboratoire de Génie Chimique. He has obtained his
Master's degree in Materials Science and Engineering at Université of Montpellier (France) and bachelor’s degree in Materials Engineering from the Federal University
of Rio Grande do Sul (Brazil). He has experience in the synthesis and characterization of nanostructured materials with photocatalytic activities, carbon nanotubes and
thermodynamic modeling of systems using the CALPHAD method.
schorne-pinto@chimie.ups-tlse.frJuliano Schorne Pinto et al., Res. Rev. J Mat. Sci. 2017, 5:7
DOI: 10.4172/2321-6212-C1-011
















