
Page 75
Notes:
conferenceseries
.com
Volume 5, Issue 5
Res. Rev. J Mat. Sci. 2017
ISSN: 2321-6212
Advanced Materials 2017
September 07-08, 2017
September 07-08, 2017 | Edinburgh, Scotland
Advanced materials & Processing
11
th
International Conference on
Adsorptive removal of sulfur compounds using Cu
2+
-based porous coordination polymers
Masashi Morita
1
, Hidenobu Wakita
1
, Takaiki Nomura
1
, Yoichiro Tsuji
1
, Masakazu Higuchi
2
and
Susumu Kitagawa
2
1
Panasonic Corporation, Japan
2
Kyoto University, Japan
A
dsorptive removal of toxic substances is of crucial importance both in industry and in living environments. In general, porous
materials such as zeolites and activated carbons are used as the adsorbents. Ag-Y zeolite is widely used for the adsorptive removal
of sulfur compounds from natural gas at ambient temperatures in fuel cell cogeneration systems in spite of its low adsorption capacity
and high cost. For the purpose of a more efficient and simple process, new types of porous materials for adsorbents are strongly
required. In recent years, porous coordination polymers (PCPs) or metal‒organic frameworks (MOFs), which have high surface
areas and act as molecular sieves owing to their micropores have been studied due to their potential applications in gas storage,
molecular separation and catalysis. PCP/MOFs, as adsorbents, offer the advantages of having high surface area, ordered structures,
and adjustable chemical functionality. The unique adsorptive reactions of various materials have been reported. Herein, we applied
porous coordination polymers (PCPs) to adsorbents for the removal of sulfur compounds and also investigated how the metal ions
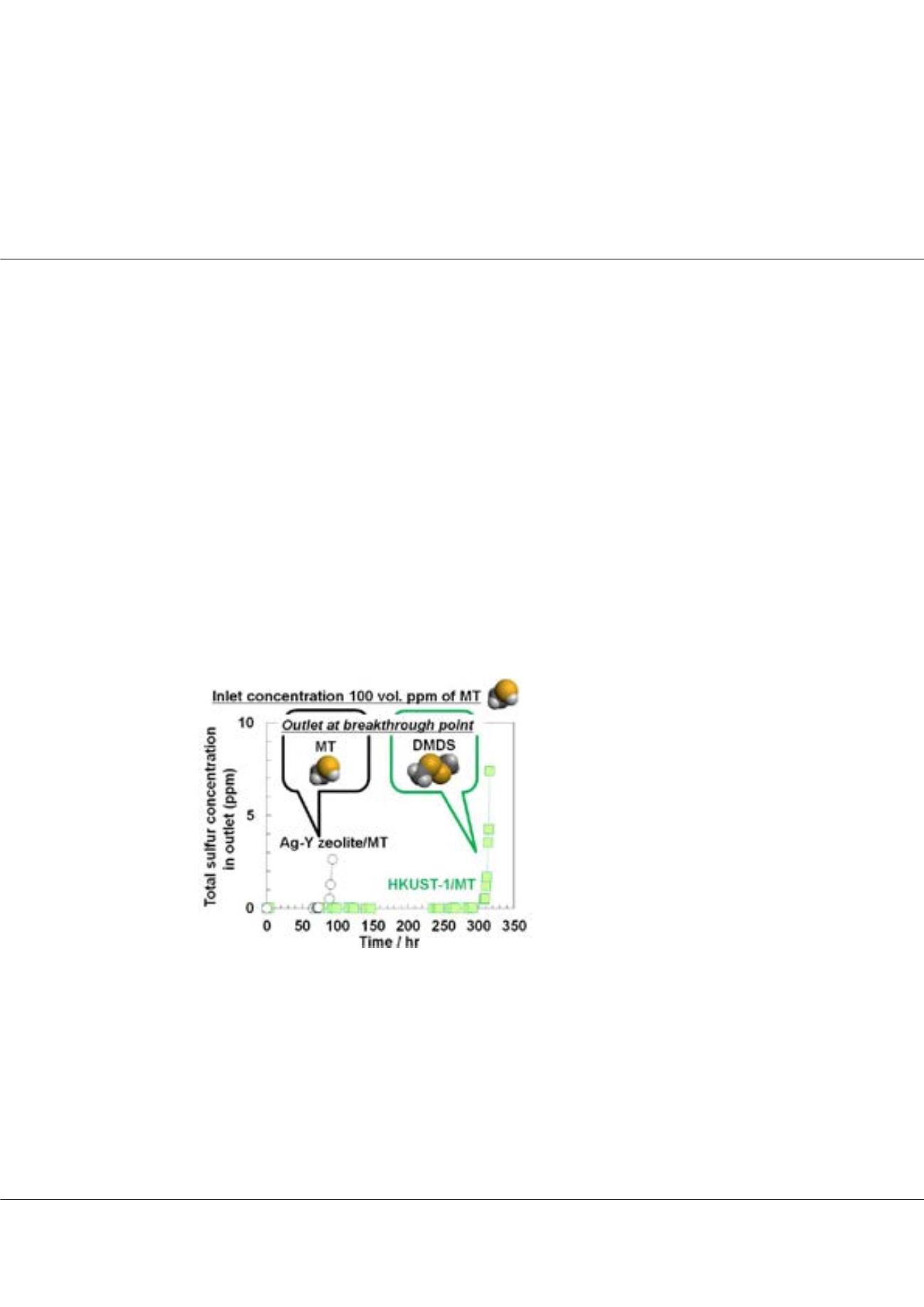
highly dispersed in PCPs affected the adsorption capacity and how the open metal sites functioned as the adsorption sites. HKUST-1,
which is composed of Cu (II) and trimesate, was examined to remove methanethiol (MT) from hydrocarbon gas at 30 °C. As a
result, HKUST-1 showed high sulfur adsorption capacities for MT (8.4 wt.%), compared with those on Ag-Y zeolite (3.0 wt.%).
Spectroscopic study revealed that a MT was adsorbed on Cu (II) site to produce a dimerized dimethyldisulfide (DMDS) accompanied
by a reduction of Cu (II) to Cu (I). To conclude, we have utilized HKUST-1 for the adsorptive removal of MT from hydrocarbon
gas. It was experimentally shown that highly dispersive Cu (II) sites in HKUST-1 are effective for the removal of sulfur compounds.
Biography
Masashi Morita was born at Saitama, Japan, in 1988. He received his
B.Sc.and
M.Sc. degrees from Waseda University in 2011 and 2013 respectively under
the direction of Professor Makoto Ogawa. He joined in Research & Development division, Panasonic Corporation as a researcher, in 2017, he became a senior
researcher. His research interests include the synthesis and applications of porous materials towards the adsorbents and catalysts.
morita.masashi1008@jp.panasonic.comMasashi Morita et al., Res. Rev. J Mat. Sci. 2017, 5:5
DOI: 10.4172/2321-6212-C1-005
Fig1:
Breakthrough curves of total
sulfur concentration for MT adsorption
















