
Volume 5, Issue 5
Res. Rev. J Mat. Sci. 2017
ISSN: 2321-6212
Advanced Materials 2017
September 07-08, 2017
Page 22
conference
series
.com
September 07-08, 2017 | Edinburgh, Scotland
Advanced materials & Processing
11
th
International Conference on
Haruo Sugi, Res. Rev. J Mat. Sci. 2017, 5:5
DOI: 10.4172/2321-6212-C1-004
Direct recording of myosin head power and recovery strokes in hydrated myosin filaments provides
evidence against the swinging lever arm mechanism in muscle contraction
M
uscle contraction results from relative sliding between actin and myosin filaments, which in turn is caused by cyclic
attachment and detachment between myosin head extending from myosin filaments and active sites on actin filaments.
A myosin head consists of catalytic (CAD), converter (COD), and lever arm (LD) domains, and connected to myosin filament
backbone via subfragment-2. Based on crystallographic and electron microscopic studies on static structures of myosin heads
and acto-myosin complex, it has been proposed that myosin head exerts power stroke by active rotation of CAD around CD,
coupled with ATP hydrolysis. This mechanism is called “swinging lever arm mechanism”, and now appears in every textbook
as a dogma explaining molecular mechanism of muscle contraction. Using the gas environmental chamber, in which hydrated
biomolecules can keep their function in the electron microscope, we succeeded in recording ATP-induced power and recovery
strokes of myosin heads, which are position-marked with two different antibodies, attaching to junctional peptide between 50k
and 20k segments of myosin heavy chain in CAD(antibody 1), and to reactive lysine residue in COD (antibody 2), respectively.
Although antibody 1 covers two main myosin-binding sites on actin to inhibit formation of actin-myosin linkages, it has no
effect on both Ca
2+
-activated muscle fiber contraction and in vitro actin-myosin sliding. On the other hand, antibody 2 shows
no effect on muscle fiber contraction, but completely inhibits in vitro actin-myosin sliding. These findings, together with our
success in recording power stroke of myosin heads position-marled with antibodies 1 and 2, constitute evidence against the
dogma (or textbook view) that (1) during muscle contraction, myosin heads do not pass through rigor configuration, and (2)
muscle contraction does not results from active rotation of CAD around COD.
Biography
Haruo Sugi graduated from postgraduate School in the University of Tokyo, Japan, with a PhD degree in 1962, and was appointed instructor in the Department of Physiol-
ogy in the University of Tokyo. From 1965 to 1967, he worked at Columbia University as a research associate, and at the National Institutes of Health as a visiting scientist.
He was a professor and Chairman in the Department of Physiology, Teikyo University Medical School, Japan, from 1973 to 2004, when he became an emeritus professor.
Sugi was also chairman of the muscle committion in the International Union of Physiological Sciences (IUPS) from 1998 to 2008.
sugi@kyf.biglobe.ne.jpHaruo Sugi
Teikyo University Medical School, Japan
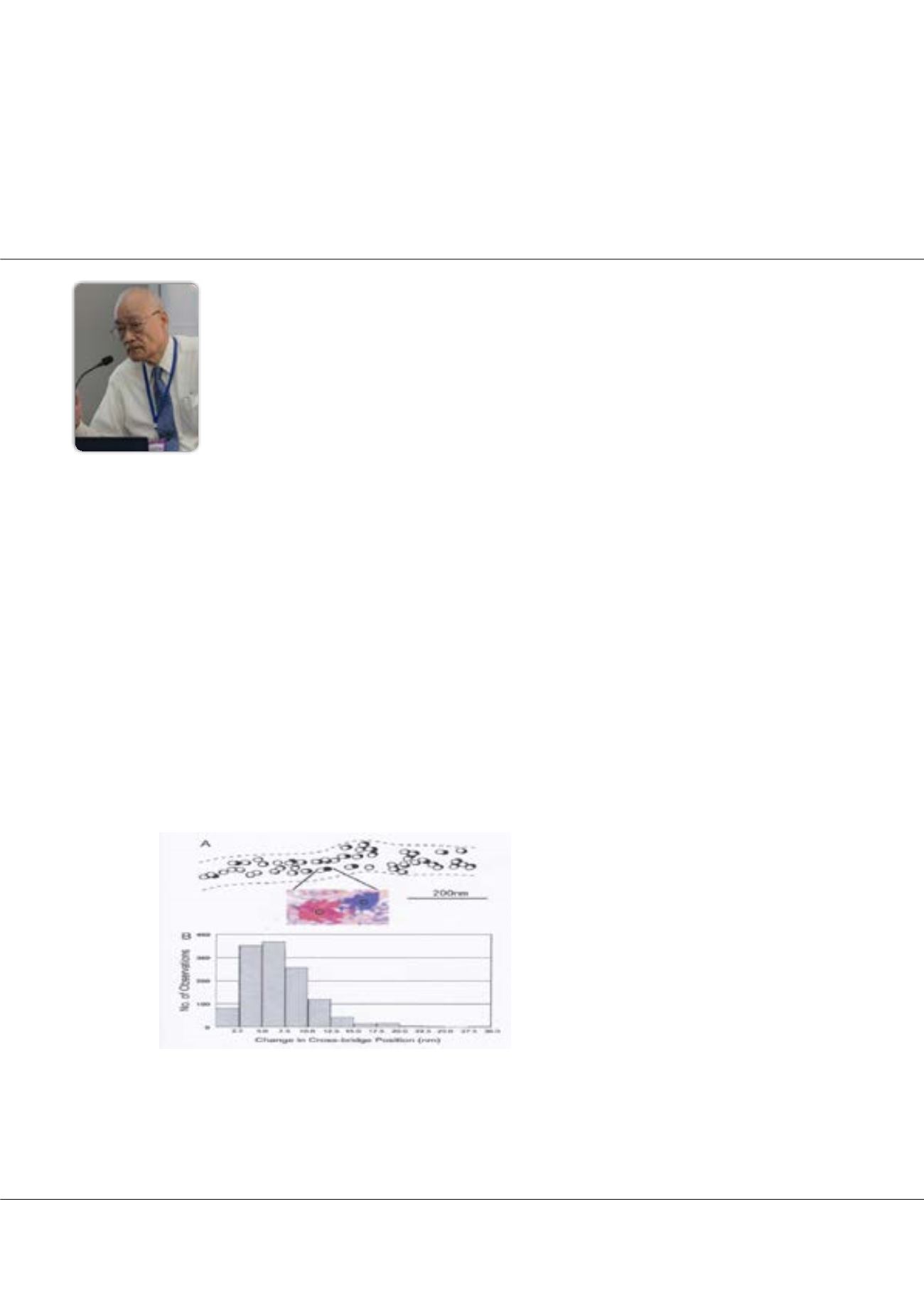
Figure 1:
ATP-induced myosin head recovery stroke in the
absence of actin filament. Open and filled circles (diameter,
20nm) show the position of gold particles, attached to myo-
sin heads with antibody 1) before and after ATP application,
respectively. (Inset) Enlarged view showing the position of
gold particle before (red) and after (blue) ATP application. (B)
Histogram showing amplitude distribution of ATP-induced my-
osin head recovery stroke.
















