
Page 105
conferenceseries
.com
Volume 6
Research & Reviews: Journal of Material Sciences
ISSN: 2321-6212
Advanced Materials 2018
September 04-06, 2018
September 04-06, 2018 | Zürich, Switzerland
21
st
International Conference on
Advanced Materials & Nanotechnology
Engineering protein interfaces yields ferritin disassembly and reassembly under benign experimental
conditions
Hai Chen
China Agricultural University, China
F
erritin is a class of naturally occurring iron storage protein; it usually consists of 24 subunits that form a hollow protein shell
with high symmetry. Recently, scientists have subverted these nature functions and used reversibly self-assembled property of
apoferritin cage controlled by pH for the encapsulation and delivery of bioactive nutrients or anticancer drug. In all these cases, the
ferritin cages shield their cargo from the influence of external conditions and provide a controlled microenvironment. However,
since ferritin disassociation generally needs extreme acidic condition (pH≤2), this strategy is limited to the structures of bioactive
compounds that are unstable at such low pH. Here, we engineered protein interfaces to yield ferritin nano cages which disassemble
at pH 4.0 and reassemble at pH 7.5. During this process, bioactive molecules can be encapsulated within protein cavity. Thus, this
engineered protein has the potential to be exploited as an alternative nano carrier for pH-sensitive bioactive compounds or drugs.
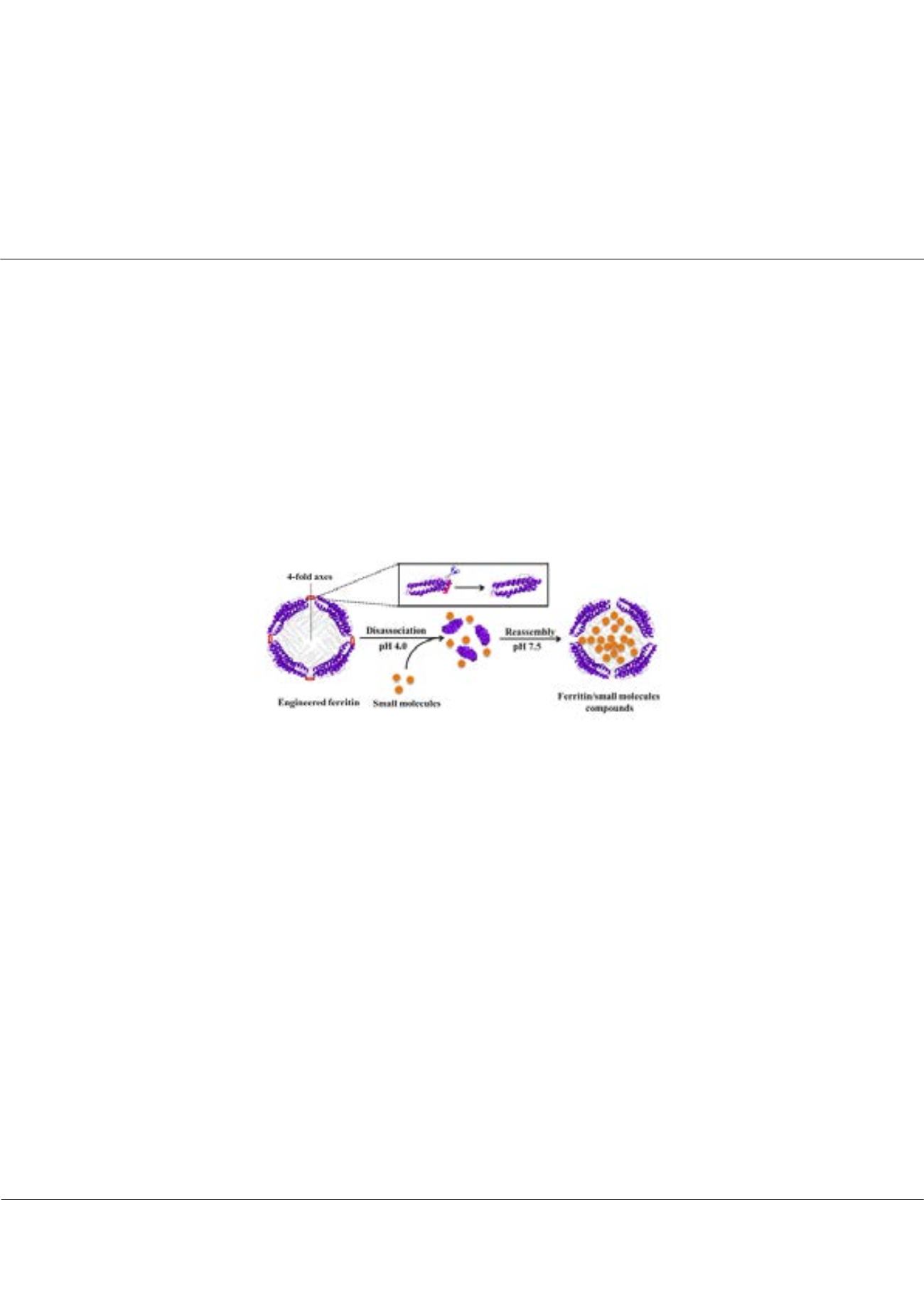
Figure:
Schematic description of preparation of an engineered ferritin and its application in encapsulating small molecules by its reversible
disassociation/reassembly. This new protein can be dissociated into subunits at pH 4.0, followed by reassembly into protein nano cage at pH 7.5
Recent Publications
1. Chen H, Zhang S, Xu C and Zhao G (2016) Engineering protein interfaces yields ferritin disassembly and reassembly
under benign experimental conditions. Chemical Communications 52(46):7402-7405.
2. Zang J, Chen H, Zhao G, Wang F and Ren F (2017) Ferritin cage for encapsulation and delivery of bioactive nutrients:
From structure, property to applications. Critical reviews in food science and nutrition 57(17):3673-3683.
3. Zhang S, Zang J, Chen H, Li M, Xu C and Zhao G (2017) The size flexibility of ferritin nano cage opens a new way to
prepare nanomaterials. Small 13(37):1701045- 1701051.
4. Zhang S, Zang J,WangW, ChenH, ZhangX,Wang F andZhaoG(2016) Conversionof the native 24‐mer ferritinNanocage
into its non‐native 16‐mer analogue by insertion of extra amino acid residues. Angewandte Chemie 55(52):16064-16070.
5. Zhang S, Zang J, Zhang X, Chen H, Mikami B and Zhao G (2016) Silent amino acid residues at key subunit interfaces
regulate the geometry of protein nanocages. ACS nano 10(11):10382-10388.
Biography
Hai Chen is a PhD student in College of Food Science and Nutritional Engineering at China Agricultural University, working on Engineering and Application of Protein
Nanostructure, especially Ferritin Nano Cages.
chenhai2509@163.comHai Chen, Res. Rev. J Mat. Sci. 2018, Volume 6
DOI: 10.4172/2321-6212-C3-020
















